- Пт. Апр 26th, 2024
Последняя запись
Как сделать СМС-рассылку — подробный гайд
СМС-рассылка – это эффективный способ доставить сообщение множеству людей одновременно. Она может быть полезна в различных ситуациях, начиная от информирования клиентов о новых акциях до организации оповещений в чрезвычайных ситуациях.…
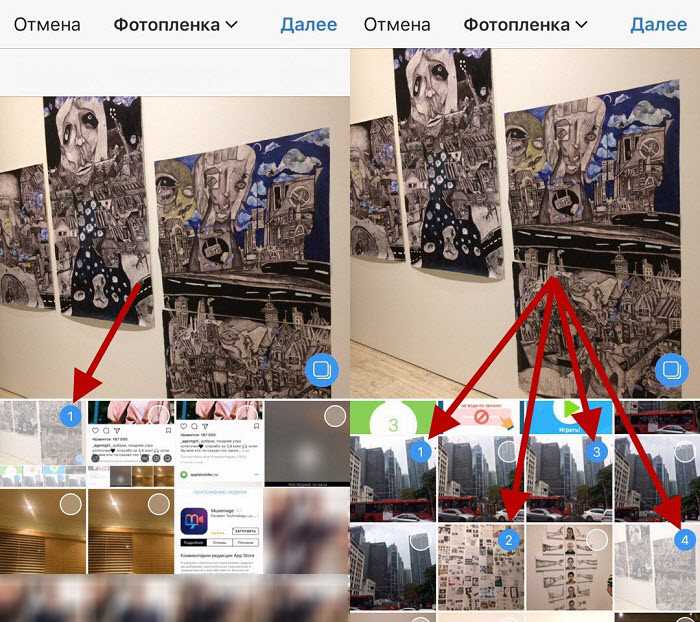
Как мгновенно добавить, загрузить и опубликовать несколько фото в Инстаграм
Инстаграм – одна из самых популярных социальных сетей, специализирующаяся на обмене фотографиями и видеозаписями. Каждый день миллионы пользователей размещают снимки, желая поделиться своими воспоминаниями, идеями или просто красивыми картинками. Но…
ТикТок и виртуальные путеводители — создание туристических видео
ТикТок – популярная социальная сеть, которая объединяет миллионы пользователей по всему миру. Она стала настоящим явлением, предлагая своим пользователям создавать краткие видеоролики и делиться ими с другими. И неудивительно, что…
Создание интернет-магазина за 5 000 рублей – миф или реальность?
Сегодня интернет-магазины становятся все более популярными, и многие предприниматели стремятся запустить свой бизнес в сети. Однако существует распространенное мнение, что можно просто так взять и создать интернет-магазин за небольшую сумму,…
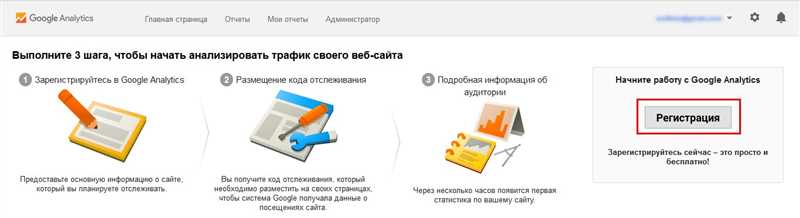
Как добавить сайт в Google Аналитика — пошаговое руководство
Google Аналитика – это мощный инструмент, который позволяет анализировать трафик на вашем сайте и получать детальную информацию о его посетителях. Чтобы начать использовать этот инструмент, первым шагом необходимо добавить свой…
Открытие ИП для интернет-магазина — обязательное условие или необязательная формальность?
В наше время интернет-магазины набирают все большую популярность. Это удобное средство для покупки товаров и услуг, которое предоставляет возможность выбирать и заказывать товары прямо из дома. Многие предприниматели задаются вопросом,…
Оптимизация страниц пагинации для SEO
SEO оптимизация страниц пагинации – это важный этап работы над сайтом, которому не хочется оказаться в индексе поисковых систем неподготовленным. Пагинация – это метод организации контента сайта по страницам, которые…
Как создать мультиязычный и мультирегиональный сайт — лучшие практики
Сложный выбор между многоязычностью и многонациональностью становится все более актуальным с развитием интернет-технологий и глобальной сети. На сегодняшний день все больше компаний и предпринимателей стремится создать веб-сайты, способные представить их…
Онлайн-запуск поисковой рекламной кампании в «Яндекс.Директе» для новичков
Яндекс.Директ – одна из крупнейших рекламных систем в России, с помощью которой можно достичь потенциальных клиентов в поисковой выдаче. Все больше и больше компаний используют данную платформу для продвижения своих…
Что такое показы в Instagram
Instagram — одна из самых популярных социальных сетей, где пользователи могут делиться своими фотографиями и видео. Вместе с тем, Instagram предлагает набор инструментов для аналитики и измерения эффективности контента. Один…